
It is the insoluble salt formed as a product of the precipitation reaction. The chemical reaction between potassium chloride (KCl) and silver nitrate (AgNO 3 ), in which solid silver chloride is precipitated out of the solution, is one of the most common examples of precipitation. When two or more two solutions containing different salts are combined, insoluble salts are produced that precipitate out of the solution. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration. The determining factors of the formation of a precipitate can vary. Precipitation reactions are an example of double displacement reactions in which a solid form residue called the precipitate is left behind. Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The insoluble salts that are formed in precipitation reactions are called precipitates. Precipitation Reaction – Definition and MeaningĪ chemical reaction that takes place in an aqueous solution where two ionic bonds combine and an insoluble salt is precipitated out as a byproduct is called the precipitation reaction. Therefore, it is referred to as the 'precipitate.’ One formed product is insoluble in the solution and is thus precipitated out. Precipitation chemistry def Precipitation weather Britannica Precipitate Definition & Meaning Coprecipitation - Wikipedia Separation by. Keeping this in mind, let us talk about Precipitation reactions in these reactions, by combining two different soluble salts in an aqueous state, two different products were formed. Some of the most common examples of chemical reactions are burning, corrosion, cooking of food and digestion etc.


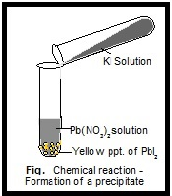
We see many chemical reactions in our day to day lives. These reactants can be in any state - solid, gaseous, or liquid. Various compounds during the chemical reaction will interact with each other and are called reactants. Interaction of reactants and the products that are involved in a chemical reaction is displayed in the chemical equations. Chemical equations are a very important tool that can help us in understanding the chemical reactions between two or more elements or compounds. During a Chemical reaction, chemical changes occur that lead to the formation of new compounds under some specific conditions.


 0 kommentar(er)
0 kommentar(er)
